Brain Machine Interfaces
Brain Machine Interfaces are an emerging set of technologies that aim to establish robust communication between the brain and the increasingly complex world around us. The following are some of the projects in our laboratory that focus on developing and advancing Brain Machine Interfaces:
Cesar Marquez-Chin, Milos R Popovic
In the development of brain-machine interfaces (BMI), operant conditioning of neural activity is a strategy that has recently re-emerged from the need to design BMI systems that feel natural and intuitive to the user. In this strategy, a single cortical neuron is conditioned to produce an activity pattern that determines when the BMI is triggered.
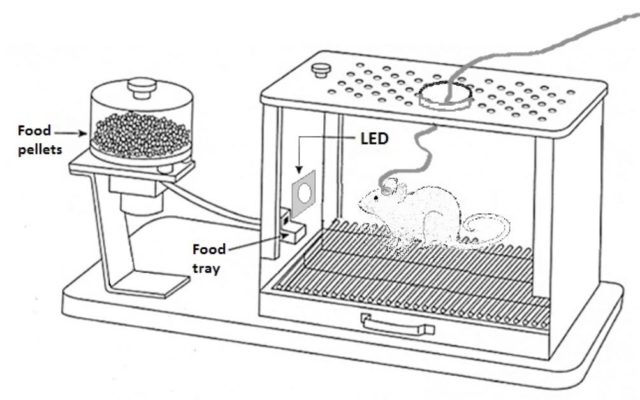
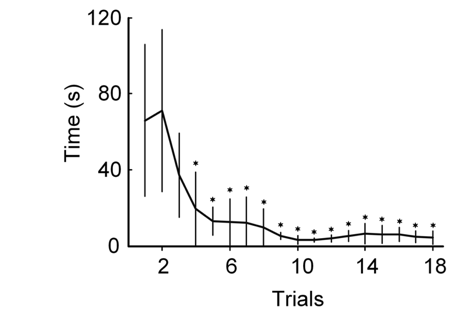
Rats are implanted with an electrode array in the neocortex (top Figure) and trained to trigger a reward dispenser by activating a single cortical neuron at progressively higher firing rates, resulting in a typical learning curve (bottom Figure). Neurons are then classified into subgroups based on their electrophysiological characteristics.
The purpose of this project is to find neurons that perform reliably in a given BMI task. In addition, operant conditioning of single neuron activity facilitates BMI implementation and usability.
Project Publications
- Garcia-Garcia MG, Marquez-Chin C, Popovic MR. Operant conditioning reveals task-specific responses of single neurons in a brain–machine interface. J Neural Eng. 2021;18(4):045003. doi:10.1088/1741-2552/abeeac
- Garcia-Garcia MG, Marquez-Chin C, Popovic MR. Operant conditioning of motor cortex neurons reveals neuron-subtype-specific responses in a brain-machine interface task. Sci Rep. 2020;10(1):19992. doi:10.1038/s41598-020-77090-2
- M. G. Garcia-Garcia et al., “Neuron-Type-Specific Utility in a Brain-Machine Interface: a Pilot Study,” The Journal of Spinal Cord Medicine, vol. 0, no. 0, pp. 1–8, Sep. 2017.
BCI-controlled FES therapy for restoration of upper limb function
Cesar Marquez-Chin, Milos R Popovic
Recent evidence suggest that the integration of brain-computer interacting technologies into the motor rehabilitation after paralysis can result in greater and/or faster outcomes.
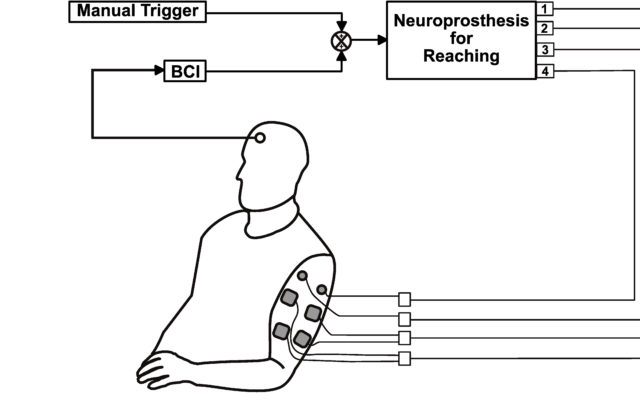
We are currently developing and testing the efficacy of functional electrical stimulation therapy (FEST), triggered by the activity of the brain, to restore voluntary movement of the arm and hand after spinal cord injury and stroke. Using brain-computer interfacing technology we identify a person’s intention to move the arm and/or hand by analyzing the electroencephalographic (EEG) activity. Immediately after this, we trigger a complex sequence of electrical pulses applied to different muscles to produce the intended action. The use of electrical stimulation is reduced gradually as the ability to move is restored and it is discontinued completely at the end of this short-term therapeutic intervention.
Our first results have allowed us to see significant improvements in even cases that typically are unable to participate in other forms of therapy.
Project Publications
C. Marquez-Chin, A. Marquis, and M. R. Popovic, “EEG-Triggered Functional Electrical Stimulation Therapy for Restoring Upper Limb Function in Chronic Stroke with Severe Hemiplegia,” Case Reports in Neurological Medicine, vol. 2016, pp. 1–11, 2016.
Deep Brain Stimulation
Neuronal response properties to electrical stimulation in movement disorder patients
Milos R Popovic
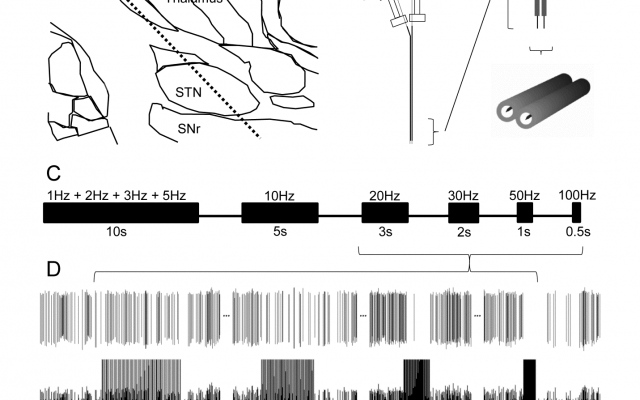
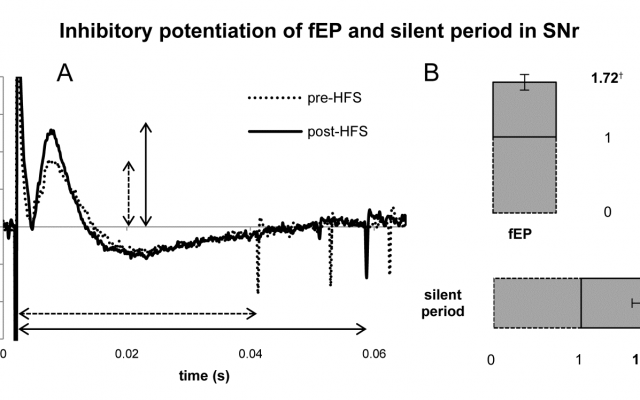
Deep brain stimulation (DBS) is an effective therapy for treating the motor symptoms of Parkinson’s disease, essential tremor, dystonia, and other movement disorders. It consists of surgically implanting chronic, indwelling electrodes into specific target structures of the brain to deliver continuous electrical stimulation. The mechanisms of action of how DBS works are varied, complex, and remain unclear. The purpose of this study is to guide the advancement and optimization of this therapy, and also to broaden its applicability. In awake surgical patients undergoing DBS implantation, we record activity from individual neurons in specific brain target structures, and can characterize their responses to electrical stimulation.
We have found that different brain structures have unique responses to stimulation that are dependent on their underlying anatomy and physiology, thus stimulation parameters cannot be programmed homogenously. Additionally, we’ve found that an important mechanism of action of therapeutic, high-frequency stimulation is neurotransmitter depletion and we’ve also discovered that therapeutic levels of high-frequency stimulation enhance synaptic plasticity.
Project Publications
L. Milosevic, S.K. Kalia, M. Hodaie, A.M. Lozano, A. Fasano, M.R. Popovic, W.D. Hutchison, “Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease,” Brain, 141(1) pp: 177-190, 2018. DOI: 10.1093/brain/awx296